Field Medical First-in-Human 6-Month Data Selected for Late-Breaking Clinical Trial at VT Symposium 2025

Three sessions feature new outcomes, a live case, and preclinical research with the FieldForce™ Ablation System.
CARDIFF-BY-THE-SEA, Calif., and PHILADELPHIA, Oct. 9, 2025 /PRNewswire/ -- Field Medical, Inc., a leader in pulsed field ablation (PFA) technology for ventricular tachycardia (VT), today announced its participation at the 20th Annual International Symposium on Ventricular Arrhythmias (VT Symposium), taking place Oct. 10 –11 in Philadelphia.
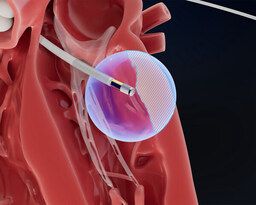
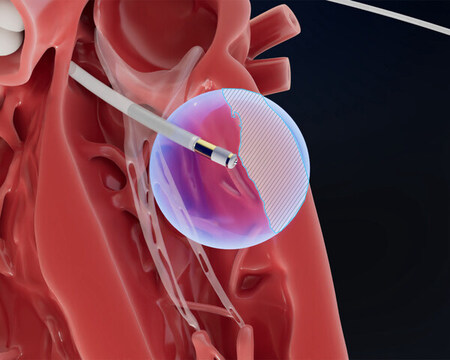 Field Medical: Engineered to reach the epicardium from the endocardium, the FieldForce™ Ablation System catheter is designed to enable focal pulsed field ablation for full-thickness, transmural lesions.
Field Medical: Engineered to reach the epicardium from the endocardium, the FieldForce™ Ablation System catheter is designed to enable focal pulsed field ablation for full-thickness, transmural lesions.
Field Medical will be featured across three sessions, including first-in-human six-month outcomes from its FieldForce™ Ablation System, the first focal contact-force PFA platform designed for VT, a pre-recorded live VT case and translational preclinical research.
Field Medical Scientific Presentations at VT Symposium
In the Late-Breaking Clinical Trial Competition, Vivek Reddy, MD, will present "High-Voltage Focal Pulsed Field Ablation to Treat Scar-Related Ventricular Tachycardia: Final 6-Month Outcomes of the First-in-Human VCAS Trial" on Friday, Oct. 10, 3:50–4:00 p.m.
On Saturday, Oct. 11, 9:30–9:40 a.m., Cory Tschabrunn, PhD, will deliver "High Voltage PFA for VT: Insights from Porcine Model of Chronic Infarction."
Later that day, in the Pre-Recorded Live Case Demonstrations, Vivek Reddy, MD, will illustrate "Image Guided High Voltage VT Ablation" from 12:35–1:55 p.m.
"The VT Symposium is a critical venue for advancing clinical science and Field Medical is proud to share meaningful new data that underscores the urgent need for innovation to help patients with VT. Our six-month outcomes demonstrate the potential of our PFA technology in treating these challenging arrhythmias, highlighting both the strength and uniqueness of our approach," said Mark Wisniewski, chairman of the board at Field Medical.
Field Medical also announced leadership changes to strengthen its focus on innovation and strategy. Steven Mickelsen, MD, founder, is now chief technology officer (CTO), leading clinical innovation and development of the FieldForce Ablation System. Kit Schneider has been appointed chief strategy officer (CSO), responsible for long-term corporate strategy, partnerships, and growth.
"Field Medical has made remarkable progress in bringing pulsed field ablation into the ventricle," Schneider said. "My focus will be on positioning the company for the next stage of growth, strengthening partnerships, and ensuring we are ready to deliver these innovations to physicians and patients worldwide."
About Field Medical® Inc.
Founded in 2022, Field Medical is a clinical-stage medical technology company committed to advancing pulsed field ablation (PFA) solutions for complex cardiac arrhythmias. Its FieldForce™ Ablation System integrates a focal catheter design with proprietary FieldBending™ energy designed to safely deliver efficient, precise ablation with the goal of improving outcomes in ventricular and atrial arrhythmia treatment. In 2024, Field Medical earned Breakthrough Device Designation and gained entry into the FDA TAP Pilot Program for its ventricular tachycardia indication.
For more information, visit www.fieldmedicalinc.com and follow us on LinkedIn, X and YouTube.
The FieldForce™ Ablation System is an investigational device and is limited by federal (or United States) law to investigational use.
Media Contact
Holly Windler
619.929.1275
holly.windler@gmail.com
Photo - https://mma.prnasia.com/media2/2792233/Field_Medical_FieldForce_Ablation_System_PFA_Catheter.jpg?p=medium600
Logo - https://mma.prnasia.com/media2/2574173/Field_Medical_Logo_Block_WhiteOnBlack_Logo.jpg?p=medium600
PR Newswire Asia Ltd.

PR Newswire
1954年に設立された世界初の米国広報通信社です。配信ネットワークで全世界をカバーしています。Cision Ltd.の子会社として、Cisionクラウドベースコミュニケーション製品、世界最大のマルチチャネル、多文化コンテンツ普及ネットワークと包括的なワークフローツールおよびプラットフォームを組み合わせることで、様々な組織のストーリーを支えています。www.prnasia.com
本プレスリリースは発表元が入力した原稿をそのまま掲載しております。また、プレスリリースへのお問い合わせは発表元に直接お願いいたします。
このプレスリリースには、報道機関向けの情報があります。
プレス会員登録を行うと、広報担当者の連絡先や、イベント・記者会見の情報など、報道機関だけに公開する情報が閲覧できるようになります。