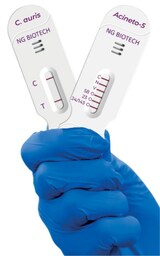
FDA Grants Breakthrough Device Designation to Two Rapid Tests Targeting Critical Drug-Resistant Pathogens

SANTA MARIA, Calif. and GUIPRY, France, Feb. 18, 2026 /PRNewswire/ -- In a major step forward against Antimicrobial Resistance (AMR), NG Biotech, in partnership with Hardy Diagnostics, announced today that the U.S. Food and Drug Administration (FDA) has granted Breakthrough Device Designations to two rapid diagnostic assays: NG-TEST®Candida auris and NG-TEST®Acineto-5®. The designation recognizes technologies with the potential to address life-threatening conditions and significant unmet medical needs.
Both tests target pathogens classified as critical priorities by the World Health Organization (WHO). Candida auris, listed in the WHO Fungal Priority Pathogens List (2022), is a multidrug-resistant yeast responsible for hospital outbreaks worldwide. It is often difficult to detect and associated with high mortality. Carbapenem-resistant Acinetobacter baumannii (CRAB), included in the WHO Bacterial Priority Pathogens List (2024), is among the most dangerous hospital-acquired bacteria due to its resistance profile and rapid transmission in healthcare settings.
NG-TEST®Candida auris is the first rapid lateral flow immunoassay specifically designed to identify C. auris from cultured samples in 15 minutes. Published data demonstrate 100% concordance with reference methods across diverse isolates, supporting its role in outbreak investigation and infection control.
NG-TEST®Acineto-5® detects and differentiates five major carbapenemase families—OXA-23-like, OXA-24/143-like, OXA-58-like, VIM, and NDM—directly from Acinetobacter samples, also delivering results within 15 minutes. The PCR-free assay is designed for ease of use, without specialized equipment.
"These breakthrough designations validate both the technology behind our assays and the real-world need they address," said Milovan Stankov-Pugès, CEO, NG Biotech.
"The designation underscores the growing urgency around rapid detection of multidrug-resistant organisms that pose serious risks in healthcare settings," said Andre Hsiung, Chief Scientific Officer of Hardy Diagnostics.
Developed and manufactured in France by NG Biotech, the assays are distributed exclusively in the United States by Hardy Diagnostics. They are currently available for Research Use Only while FDA review continues.
By accelerating detection of high-risk pathogens, these breakthrough-designated tests aim to strengthen surveillance, guide infection control decisions, and support global efforts to fight antimicrobial resistance.
Learn more about NG Biotech
Learn more about Hardy Diagnostics
Media & Contact Information
NG BIOTECH
Atelier Relais Le Tremplin
Parc d'Activités de Courbouton, Secteur 1
35480 Guipry, France
+33 (0)2 23 30 17 83
Ms. Pauline Cognet
p.cognet@ngbiotech.com
www.ngbiotech.com
HARDY DIAGNOSTICS
1430 West McCoy Lane
Santa Maria, CA 93455, USA
(805) 346-2766 ext. 5598
Ms. Megan Maloney Roesner
RoesnerM@hardydiagnostics.com
www.HardyDiagnostics.com
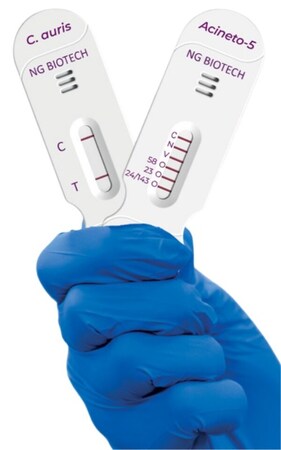 Image of NG-TEST® Candida auris and NG-TEST® Acineto-5® LFA Cassettes
Image of NG-TEST® Candida auris and NG-TEST® Acineto-5® LFA Cassettes
PR Newswire Asia Ltd.

PR Newswire
1954年に設立された世界初の米国広報通信社です。配信ネットワークで全世界をカバーしています。Cision Ltd.の子会社として、Cisionクラウドベースコミュニケーション製品、世界最大のマルチチャネル、多文化コンテンツ普及ネットワークと包括的なワークフローツールおよびプラットフォームを組み合わせることで、様々な組織のストーリーを支えています。www.prnasia.com
本プレスリリースは発表元が入力した原稿をそのまま掲載しております。また、プレスリリースへのお問い合わせは発表元に直接お願いいたします。
このプレスリリースには、報道機関向けの情報があります。
プレス会員登録を行うと、広報担当者の連絡先や、イベント・記者会見の情報など、報道機関だけに公開する情報が閲覧できるようになります。