Gan & Lee Pharmaceuticals to Present Groundbreaking Data on Three Innovative Products at the American Diabetes Association's 84th Scientific Sessions

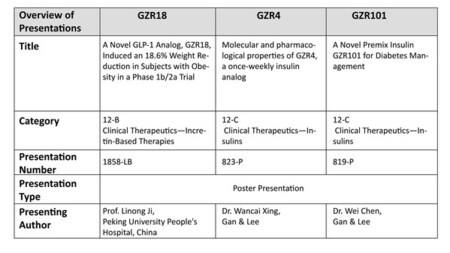
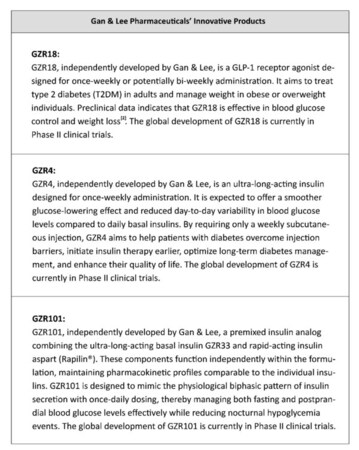
A phase 1b/2a trial evaluated once- and bi-weekly GZR18, a novel GLP-1 analog, in Chinese subjects with obesity/overweight
Pre-clinical studies evaluated the molecular and pharmacological properties of GZR4, an investigational once-weekly insulin analog
Pre-clinical studies evaluated and characterized an ultra-long-acting basal insulin, GZR33, and its premixed formulation GZR101
BRIDGEWATER, N.J., June 18, 2024 /PRNewswire/ -- Gan & Lee Pharmaceuticals (Shanghai Stock Exchange: 603087) announced today the presentation of three abstracts featuring their investigational products at the American Diabetes Association's (ADA's[1]) 84th Scientific Sessions. The meeting will be held in-person and virtually from June 21-24, 2024, in Orlando, Florida .
All abstracts will be published on the journal Diabetes® website. Data from the selected studies will be presented live on June 22, from 12:30-1:30 PM EDT in the Poster Hall.
The above abstracts and presentations represent the data that will be showcased or published by Gan & Lee. This press release includes forward-looking statements regarding investigational products currently in development by Gan & Lee. It is important to note that there are risks associated with drug development, and there is no guarantee that future studies will produce results consistent with those presented at the ADA's 84th Scientific Sessions.
References:
[1]. ADA's 84th Scientific Sessions. https://professional.diabetes.org/scientific-sessions
[2]. Zhang M, Zhang Y, Peng X, et al. GZR18, a novel long-acting GLP-1 analog, demonstrated positive in vitro and in vivo pharmacokinetic and pharmacodynamic characteristics in animal models. Eur J Pharmacol. 2022;928:175107. doi:10.1016/j.ejphar.2022.175107
About Gan & Lee
Gan & Lee Pharmaceuticals developed the first Chinese domestic insulin analog. Currently, Gan & Lee has six core insulin products, including five insulin analog varieties: long-acting glargine injection (Basalin®), fast-acting lispro injection (Prandilin™), fast-acting aspart injection (Rapilin®), mixed protamine zinc lispro injection (25R) (Prandilin™25), aspart 30 injection (Rapilin®30), and one human insulin injection - mixed protamine human insulin injection (30R) (Similin®30). The company has two approved medical devices in China, namely reusable insulin injection pen (GanleePen), and disposable pen needle (GanleeFine®).
In China's second Volume Based Procurement (VBP) in 2024, Gan & Lee Pharmaceuticals ranked second overall and first among domestic companies in terms of procurement demand for insulin analogs. The company is also making strides in international markets, with the disposable pen needle (GanleeFine®) approved by the US Food and Drug Administration (FDA) in 2020 and received GMP inspection approval from the European Medicines Agency (EMA) in 2024. These achievements significantly boost Gan & Lee's competitiveness in both international and domestic markets.
In the future, Gan & Lee will strive for comprehensive coverage in diabetes treatment. Moving forward with its mission to become a world-class pharmaceutical company, Gan & Lee will also actively develop new chemical entities and biological drugs, focusing on treatments for metabolic diseases, cardiovascular diseases, and other therapeutic areas.
Further Information
BPRD@ganlee.com(Media)
BD@ganlee.com(Business Development)
PR Newswire Asia Ltd.

PR Newswire
1954年に設立された世界初の米国広報通信社です。配信ネットワークで全世界をカバーしています。Cision Ltd.の子会社として、Cisionクラウドベースコミュニケーション製品、世界最大のマルチチャネル、多文化コンテンツ普及ネットワークと包括的なワークフローツールおよびプラットフォームを組み合わせることで、様々な組織のストーリーを支えています。www.prnasia.com
本プレスリリースは発表元が入力した原稿をそのまま掲載しております。また、プレスリリースへのお問い合わせは発表元に直接お願いいたします。
このプレスリリースには、報道機関向けの情報があります。
プレス会員登録を行うと、広報担当者の連絡先や、イベント・記者会見の情報など、報道機関だけに公開する情報が閲覧できるようになります。













