Taiwan's Antrodia Cinnamomea Charging Towards the International Market: Sustainable Development and Diverse Research on Antrodia cinnamomea
TAIPEI, May 31, 2024 /PRNewswire/ -- The U.S. Food and Drug Administration (FDA) recently responded to the safety evaluation of Antrodia cinnamomea mycelium, submitted by Greenyn Biotechnology for a New Dietary Ingredient (NDI) with a "no objection" letter. It has instilled confidence in the integration of Taiwan's national treasure into the international market.
During the recently held "International Symposium on Taiwan Medicinal Mushroom: Antrodia cinnamomea (ISTAC)," dozens of experts and scholars presented research validating the liver-protective, hangover-alleviating, detoxifying functions of Antrodia cinnamomea. Additionally, the high safety profile of Antrodia cinnamomea mycelium in clinical applications for patients with Hepatitis B was highlighted and expected to increase its international recognition. Moreover, enable it to enter the multi-billion-dollar herbal dietary supplement market in the United States.
Cultivating "Sustainable Antrodia cinnamomea"
Wild Antrodia cinnamomea contains over 200 bioactive components and holds high value for health benefits. To date, over 30 types of triterpenoids and steroidal saponins have been isolated. In addition to triterpenoids, polysaccharides, derivatives of malic acid, derivatives of succinic acid, quinones, diterpenes, and more exhibit significant physiological effects. The diversity and effectiveness of these active components are remarkable. Due to the rarity of the bull camphor tree, logging is prohibited. This has led producers to employ various Antrodia cinnamomea production methods based on differences in research techniques and resource allocation (see Table 1). These range from cultivating fruiting bodies in basswood, which closely mimics the composition of wild Antrodia cinnamomea, to solid-state cultivation of mycelium, which offers a relatively cost-effective option for consumers. The development efforts of producers contribute to the sustainable inheritance of Antrodia cinnamomea's vitality.
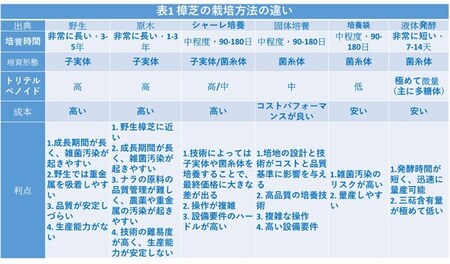 Differences in cultivation methods of Antrodia cinnamomea (Table 1)
Differences in cultivation methods of Antrodia cinnamomea (Table 1)
Diverse Research on Antrodia cinnamomea
The health benefits of Antrodia cinnamomea, including liver protection, immune enhancement, and anticancer properties, have been well-established. Recent attention has also been drawn to its applications in combating Hepatitis B, as well as its detoxification and hangover relief effects related to daily life:
Antiviral Effects Against Hepatitis B
According to estimates by the Ministry of Health, Labour and Welfare, Japan adds approximately 5,000 new cases of acute hepatitis B each year. On the other hand, since 70-80% of transient infections eventually remain asymptomatic, the annual number of HBV infections is estimated to be around 20,000 people. The number of deaths annually due to hepatitis B virus-related liver cancer is approximately 5,000 people, while the estimated number of deaths from cirrhosis is around 1,000 people.
Cell experiments analyzing the antiviral capabilities against Hepatitis B reveal that the patented solid-state cultured mycelium of Antrodia cinnamomea can inhibit the surface antigen and viral DNA replication of Hepatitis B by as much as 12% and 35% of the dosage of the Hepatitis B drug Lamivudine, respectively.
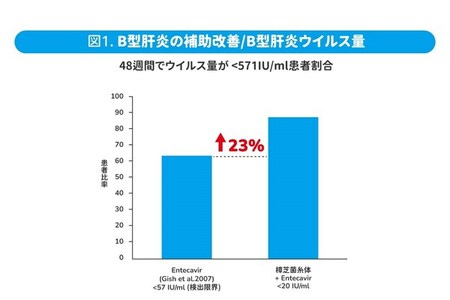
Clinical studies in humans further demonstrate that supplementation with Antrodia cinnamomea mycelium, in conjunction with the Hepatitis B drug Entecavir, over a period of 48 weeks, significantly reduces levels of GOT by 58% and GPT by 76% in patients undergoing Hepatitis B treatment. Moreover, this combined treatment has shown no adverse effects on indicators such as liver function, kidney function, blood, and urine, indicating its safety in clinical application. In comparison to using the Hepatitis B drug alone, the mycelium of Antrodia cinnamomea can assist in a 23% increase in patients achieving virus control and a 34% improvement in GPT levels (5).
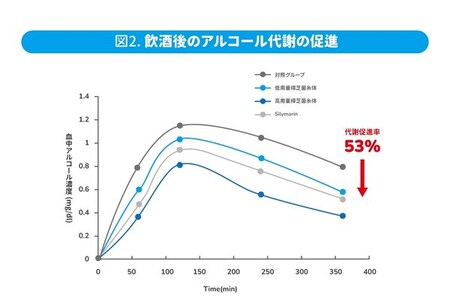
Alcohol Metabolism
In modern society, social drinking is commonplace, but the symptoms of alcohol intoxication can be off-putting. Animal experiments indicate that supplementation with Antrodia cinnamomea mycelium, whether before or 30 minutes after alcohol consumption, effectively accelerates the process of sobering up. It rapidly metabolizes alcohol in the blood by 39% and acetaldehyde by 53%, allowing individuals to wake up from their alcohol-induced state seven hours earlier. Additionally, four weeks of daily supplementation significantly enhances the activity of alcohol-metabolizing enzymes in the liver, increasing ethanol dehydrogenase activity by 40% and acetaldehyde dehydrogenase activity by 42%. This provides a viable option for the busy modern individual to alleviate the burden of alcohol consumption.
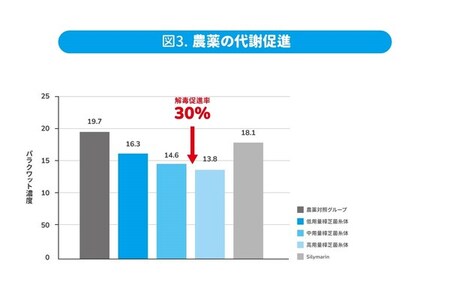
Pesticide Metabolism
The widespread use of pesticides means that toxins find their way into our bodies inadvertently. Research involving randomly supplemented rats with varying doses of Antrodia cinnamomea mycelium or the western medicine Silymarin, followed by the administration of pesticides such as Paraquat or Glyphosate the following day, revealed a notable reduction in pesticide residue levels in the blood. The results indicate that Antrodia cinnamomea mycelium can expedite the metabolism of Paraquat by 30% and Glyphosate by 48.7%. Additionally, higher doses of Antrodia cinnamomea mycelium were more effective than Silymarin in this regard.
Conclusion
The unique Antrodia cinnamomea species in Taiwan, cultivated through technological means, has achieved sustainable inheritance, avoiding harm to forest trees. Numerous researchers have diligently worked to systematically verify its health benefits. Beyond the traditional roles in immunity enhancement and cancer prevention, Antrodia cinnamomea's applications have expanded to address modern-day concerns such as chronic hepatitis, hangover relief, and detoxification. The most challenging obstacle, the issue of safety in international trade, has been surmounted by obtaining the "unreserved opinion" notification for New Dietary Ingredients (NDI) from the world-renowned U.S. FDA. This achievement marks a powerful foothold for Taiwan in the global Antrodia cinnamomea health supplement market, affirming Taiwan's prowess in biotechnology and healthcare research and development.
Reference
US FDA. New Dietary Ingredient Notification NO. 1170.
Tyler Smith et al. US Sales of Herbal Supplements Increase by 8.6% in 2019. HerbalGram (2020) 127:54-69.
Health Promotion Administration, Ministry of Health and Welfare: (2017) Prevention and treatment of liver diseases and liver cancer.
Pang-Kuei Hsu: The Diverse Benefits and International Safety Breakthroughs of Antrodia Cinnamomea. (2021) International Symposium on the Industrial Development and Clinical Applications of Antrodia Cinnamomea.
Shung-Te Kao: The Clinical Application of Antrodia Cinnamomea Mycelium as an Adjunctive Therapy for Hepatitis B. (2021) International Symposium on the Industrial Development and Clinical Applications of Antrodia Cinnamomea.
Robert G. Gish et al. Entecavir Therapy for up to 96 Weeks in Patients With HBeAg-Positive Chronic Hepatitis B. Gastroenterology (2007) 133: 1437–1444.
PR Newswire Asia Ltd.

PR Newswire
1954年に設立された世界初の米国広報通信社です。配信ネットワークで全世界をカバーしています。Cision Ltd.の子会社として、Cisionクラウドベースコミュニケーション製品、世界最大のマルチチャネル、多文化コンテンツ普及ネットワークと包括的なワークフローツールおよびプラットフォームを組み合わせることで、様々な組織のストーリーを支えています。www.prnasia.com
本プレスリリースは発表元が入力した原稿をそのまま掲載しております。また、プレスリリースへのお問い合わせは発表元に直接お願いいたします。
このプレスリリースには、報道機関向けの情報があります。
プレス会員登録を行うと、広報担当者の連絡先や、イベント・記者会見の情報など、報道機関だけに公開する情報が閲覧できるようになります。














